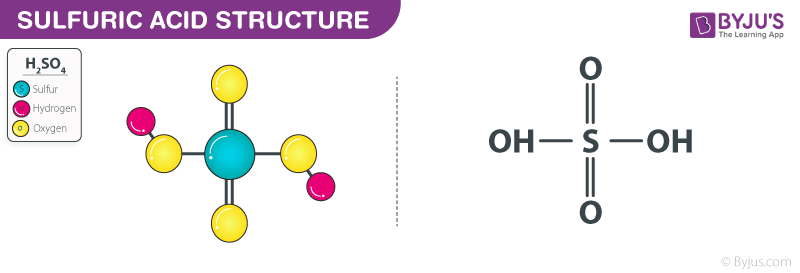
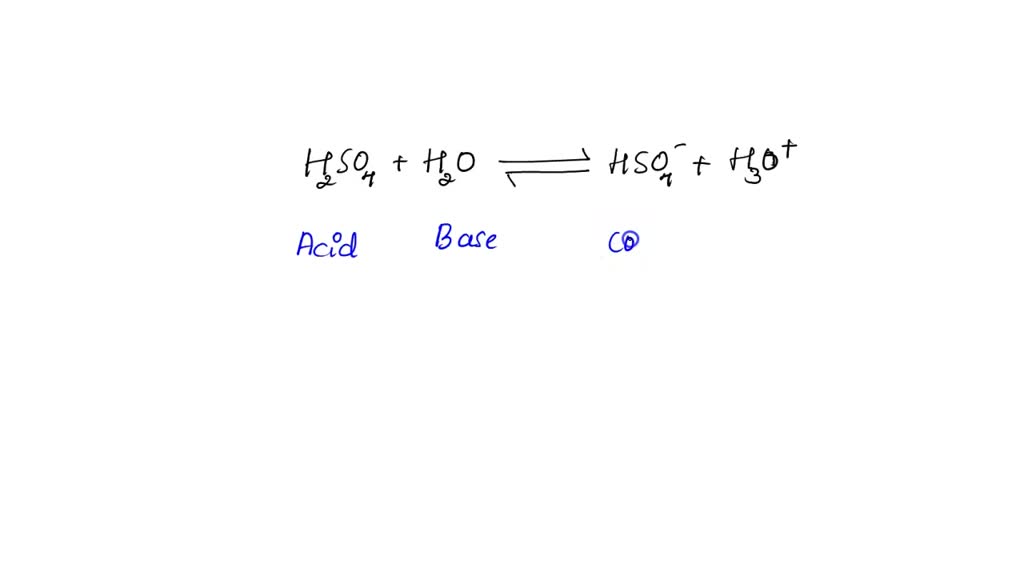Ammonia Catalyzed Formation of Sulfuric Acid in Troposphere: The Curious Case of a Base Promoting Acid Rain | The Journal of Physical Chemistry A

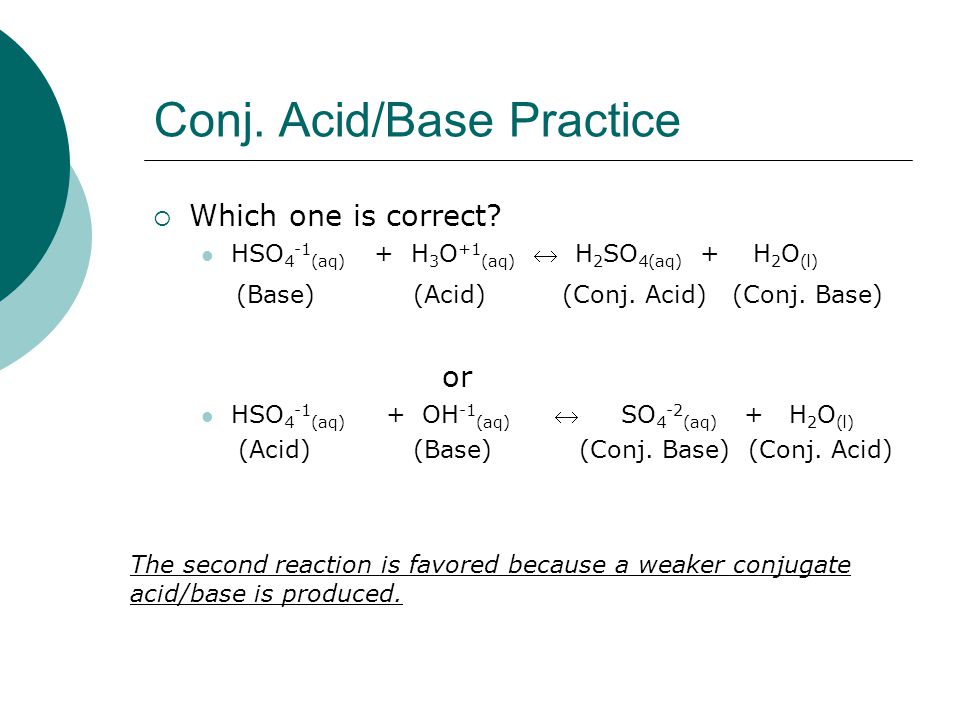
Balance KOH + H2SO4 = K2SO4 + H2O (Potassium Hydroxide and Sulfuric Acid) | Balance KOH + H2SO4 = K2SO4 + H2O (Potassium Hydroxide and Sulfuric Acid) Hello Everyone! Welcome back to

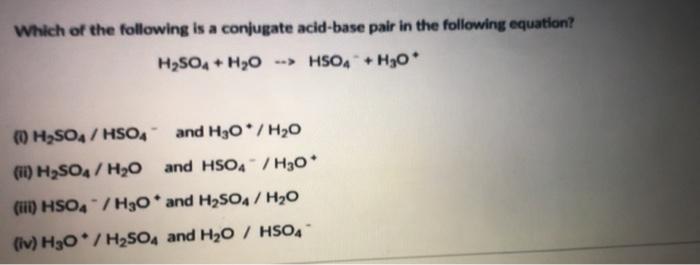
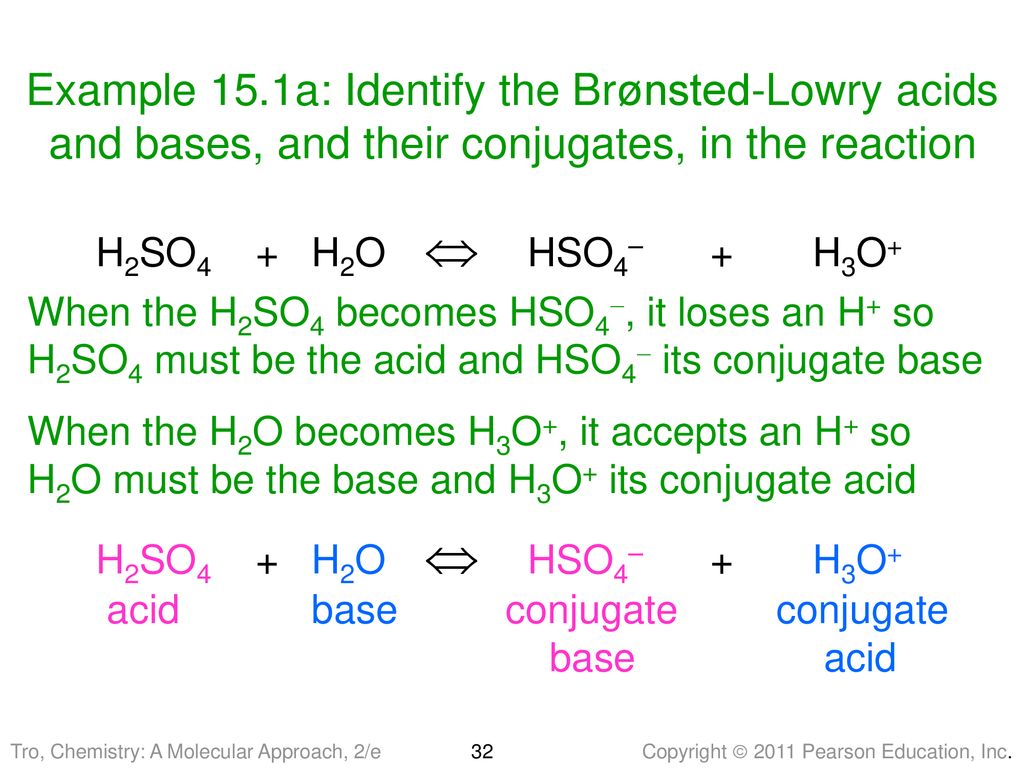
What substance is acting as the Brønsted-Lowry acid in the following forward reaction? H2SO4 + H2O - Brainly.com